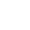Incazelo yemitha elingu-ph
Imitha ye-pH isho ithuluzi elisetshenziselwa ukunquma inani le-pH lesixazululo.Imitha ye-pH isebenza ngomgomo webhethri le-galvanic.Amandla e-electromotive phakathi kwama-electrode amabili ebhethri ye-galvanic asekelwe emthethweni we-Nerns, ongahlobene kuphela nezakhiwo zama-electrode, kodwa futhi ahlobene nokugxila kwe-hydrogen ions esixazululweni.Kunobudlelwano obuhambisanayo phakathi kwamandla e-electromotive ebhethri eliyinhloko kanye nokugxiliswa kwe-ion ye-hydrogen, futhi i-logarithm eyinegethivu yokugxiliswa kwe-ion ye-hydrogen yinani le-pH.Imitha ye-pH iyithuluzi elivamile lokuhlaziya, elisetshenziswa kakhulu kwezolimo, ukuvikelwa kwemvelo kanye nezimboni.I-pH yomhlabathi ingenye yezinto ezibalulekile eziyisisekelo zomhlabathi.Izinto ezifana nezinga lokushisa namandla e-ionic esixazululo okufanele sihlolwe kufanele zicatshangelwe ngesikhathi sokukalwa kwe-pH.
Umgomo we-ph mitha
I-pH ichazwa njenge-logarithm enegethivu yokugxiliswa kwe-hydrogen ion kusixazululo esinamanzi.Nakuba lokhu kuzwakala kuyinkimbinkimbi, ngamagama alula kakhulu, i-pH iyinombolo esetshenziselwa ukulinganisa ubumuncu noma i-alkalinity yesisombululo.Inombolo ikhombisa inani lama-ion e-hydrogen into ethile engayikhipha exazululweni.Ebangeni le-pH, i-pH engu-7 ibhekwa njengengathathi hlangothi.Izixazululo ezine-pH ka-0-7 zithathwa njenge-acidic, futhi izixazululo ezingaphezu kuka-7 kuya ku-14 zibizwa ngokuthi izixazululo ze-alkaline.Ezinhlelweni zebhayoloji, i-pH ibalulekile.Ngenxa ye-pH elungiswe ngokucophelela, iningi lama-biomolecules emzimbeni wethu lingenza imisebenzi emihle kakhulu.Ngisho nasohlelweni lokuhlola, i-pH edingekayo kufanele igcinwe ukuze kutholwe imiphumela enembile.Ngakho-ke, ekuhlolweni kwebhayoloji, idivayisi ebizwa ngokuthi imitha ye-pH isetshenziswa ukuqapha ngokucophelela i-pH.
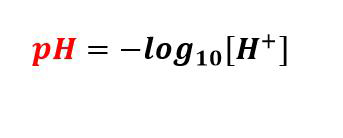
Imitha ye-pH iyi-electrode esabelayo nge-pH ekala umsebenzi wama-ion e-hydrogen esixazululweni bese idlulisela lolu lwazi.Idivayisi iqukethe amashubhu amabili engilazi, ngalinye eliqukethe i-electrode, i-electrode eyireferensi kanye ne-sensor electrode.I-electrode eyireferensi yenziwe ngesixazululo se-KCl esigcwele, kuyilapho i-electrode yenzwa iqukethe isisombululo se-buffer esine-pH engu-7, futhi ucingo olusiliva olunamekwe nge-chloride esiliva lucwiliswa kulezi zixazululo ezimbili.Ekupheleni kwe-sensor electrode i-bulb eyenziwe ngengilazi enezimbotshana eboshwe nge-silica nosawoti wensimbi.
Ukukala i-pH yesisombululo, imitha ye-pH icwiliswa esixazululweni.Ngemva kokuba isibani se-electrode yenzwa sithintana nesisombululo, ama-ion e-hydrogen asesixazululweni azothatha indawo yama-ion ensimbi ku-bulb.Lokhu kufakwa esikhundleni kwama-ion ensimbi kubangela ukugeleza kwamanje ocingweni lwensimbi, olufundwa nge-voltmeter.
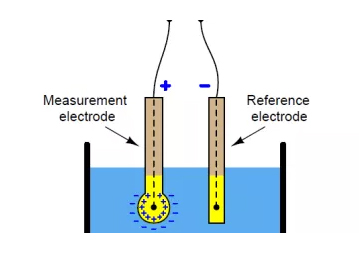
Imitha ye-pH ingenye yezinto ezisetshenziswa kakhulu kumalabhorethri ezinto eziphilayo.Hlaziya njalo i-pH yamabhafa, izixazululo nama-reagents ukuze uqinisekise ukuthi izimo zokuhlola zilungile.Ukuqinisekisa ukufundwa okunembile, okokusebenza kufanele kukalwe njalo.
Ukusetshenziswa komtshina wamamitha we-PH
Ukusetshenziswa komtshina wemitha ye-PH kunqubo yokuhlanza indle yasekhaya

Ukusetshenziswa kwemitha ye-pH ekwelashweni kwamanzi angcolile nge-electroplating

Ukusetshenziswa kwe-Online PH Meter embonini

Ukulinganiswa kwemitha elingu-PH
Isikhathi sokuthumela: Dec-15-2021